
Ninguna ciencia, en cuanto a ciencia, engaña; el engaño está en quien no sabe. (Miguel de Cervantes)

Investigadores españoles y alemanes han ideado un mecanismo para llevar agentes bioactivos al interior de la célula utilizando unos compuestos de boro que son capaces de desordenar las moléculas de agua y deshidratar la carga que llevan. De esta forma pueden atravesar la membrana celular sin dañarla y entregar el cargamento, lo que puede resultar de gran interés para administrar fármacos.

Esquemas de la actividad portadora de los nuevos compuestos de boro. Sus propiedades supercaotrópicas
les permite desordenar las moléculas de agua y deshidratar así la carga que transportan,
para poder atravesar la membrana hidrófoba y entregarla dentro. / A. Barba-Bon, G. Salluce et al./ Nature
Uno de los grandes retos en el diseño de fármacos es introducir en la célula moléculas que sean
solubles en agua, pues la membrana celular supone una barrera semipermeable que este
tipo de sustancias no pueden atravesar fácilmente. Para superarla, los expertos vienen
empleando distintos vehículos artificiales como polímeros, lípidos y algunos tipos de péptidos
que consiguen llevar su carga al interior celular con éxito.
Hasta la fecha, todos estos portadores tienen una estructura anfifílica (con un extremo afín
al agua y el otro a los lípidos), lo que les permite enmascarar de manera transitoria su carga
en un envoltorio hidrófobo para abrirse paso a través de la membrana lipídica. Pero esta
estrategia tiene sus limitaciones: en ocasiones, este mismo comportamiento puede
dañar la membrana, y en otros casos los compuestos anfifílicos muestran poca solubilidad,
lo que puede limitar su efectividad.
Estos compuestos de boro tienen propiedades supercaotrópicas que les permite desordenar
las moléculas de agua y deshidratar la carga que llevan, para así poder atravesar la
membrana celular y entregarla
Ahora investigadores del Centro Singular de Investigación en Química Biolóxica y Materiais
Moleculares (CiQUS) de la Universidad de Santiago de Compostela, en colaboración con
científicos de la Universidad Jacobs de Bremen (Alemania), han desarrollado una nueva
clase de vehículos moleculares para administrar fármacos que trasciende el dogma anfifílico.
El avance lo publican en un articulo de acceso abierto en Nature.
Los nuevos portadores son clústeres o conjuntos de átomos de boro con forma esférica,
carga negativa y una excelente solubilidad en el agua. La clave reside en su naturaleza
supercaotrópica, una propiedad que les permite desordenar las moléculas de agua y
deshidratar así la carga que transportan para poder atravesar la membrana hidrófoba.
“Hemos identificado una clase completamente nueva de vehículos que podrían ser utilizados
para llevar distintos fármacos al interior de las células. Los aniones supercaotrópicos son
una nueva herramienta, totalmente diferente a las que había hasta la fecha, para poder
internalizar sustancias hidrófilas en la célula cuyo potencial justo se acaba de
empezar a explorar”, destaca Guilia Salluce del CiQUS, una de las dos primeras coautoras
del estudio.
Javier Montenegro y Giulia Salluce, dos de los investigadores que han participado en el estudio
. / CiQUS
Por su parte, el grupo alemán, que dirige el profesor Werner Nau, ha estudiado el
comportamiento de estos cúmulos de boro, junto a otros átomos (como el hidrógeno,
el cloro, el bromo...), en modelos de membranas basados en vesículas artificiales.
Candidato óptimo de boro y bromo
En particular, se ha comprobado que un compuesto de boro y bromo (B12Br122- )
es el candidato óptimo de esta nueva clase de portadores de boro supercaotrópicos.
Interactúa con las moléculas a transportar de una manera totalmente novedosa,
sin necesidad de agregarse con ella o tener que encapsularla.
Hemos identificado una clase completamente nueva de vehículos que podrían ser utilizados
para llevar distintos fármacos al interior de las células
“Los nuevos vehículos tienen unas propiedades de transporte muy particulares”, comenta
Andrea Barba-Bon, investigadora del equipo alemán y la otra coautora del estudio,
“a diferencia de los tradicionales compuestos anfifílicos, el orden en que se añaden
los clústeres y las moléculas que queremos transportar a las vesículas, o incluso
el tipo de membrana, tienen un efecto mínimo sobre su efectividad”.
La nueva estrategia sirve para administrar con gran eficiencia una amplia variedad
de sustancias bioactivas, desde pequeñas moléculas a péptidos de mayor tamaño.
Estos complejos de boro pueden transportarlas con éxito al interior de células vivas,
como ha demostrado el grupo del ICFO, liderado por el profesor Javier Montenegro.
Los investigadores del centro gallego han conseguido llevar diferentes cargas hidrofílicas
al interior de las células, incluyendo la faloidina –una molécula empleada habitualmente
como marcador bioquímico del citoesqueleto– hasta el citosol en el interior celular, y teñir
de este modo el esqueleto intracelular de distintos tipos de células.
Los investigadores del CiQUS han llevado diferentes cargas hidrofílicos al interior de las células,
incluyendo la faloidina, una molécula empleada habitualmente como marcador bioquímico de
l citoesqueleto. / Montenegro Lab.
“Anticipamos que el amplio y distinto espectro de entrega de nuestros portadores
supercaotrópicos será el punto de partida de estudios celulares-biológicos, neurobiológicos,
fisiológicos y farmacéuticos conceptualmente distintos”, concluyen los autores en su artículo.
Referencia:
Andrea Barba-Bon, Guilia Salluce et al.“Boron Clusters as Broadband Membrane Carriers”.
Nature, 2022.

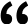
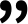
 Fuente: CiQUS
Fuente: CiQUSWhile the idea for this infographic was prompted by the eruption of crocuses currently taking place in our garden, these are Crocus vernus, the spring crocus. The crocus from which saffron is obtained is commonly called the saffron crocus, or sometimes the autumn crocus (more on that alternative later). As the latter name suggests, the saffron crocus, Crocus sativus, flowers in the autumn.
Saffron is obtained from the crocus stigmas, three deep red tendrils protruding from the centre of each flower. It takes a colossal 150 crocus flowers to produce a single gram of dried saffron, which goes a long way towards explaining why it’s the most expensive spice on supermarket shelves. Saffron’s deep red colour is due to the presence of crocin, a compound derived from the carotenoid compound crocetin. Crocin and related compounds are found in other crocuses, too, contributing to the range of yellows and oranges.
The purples and lilacs of crocus petals are due to a different group of compounds: anthocyanins. Researchers have identified nine key anthocyanin compounds as contributing to crocus colour, mainly glucosides of delphinidin and petunidin. They also identified some malonated anthocyanins which appear to be completely unique to crocuses.
While the crocus from which saffron is derived is sometimes referred to as “autumn crocus”, this moniker has the potential for deadly confusion. “Autumn crocus” is also commonly used as a name for several species in the Colchicum genus. These plants can look very similar to the saffron crocus, and also flower during the autumn, but you definitely don’t want to harvest any parts of them – all parts of the plant contain the toxic alkaloid colchicine.
Justin Brower over at Nature’s Poisons has a great post on colchicine, where he goes into the mechanism behind its toxicity:
Colchicine has two modes of action in the body. In the first, colchicine inhibits neutrophil activity. These are a type of white blood cell that kicks into gear during an immune response, causing inflammation. […] The second mode of action is by binding to tubulin, which in turn inhibits mitosis […] the process in the cell cycle in which the chromosomes are split into two identical daughter cells. We need mitosis for growth and replacement.
Colchicine: Don’t Eat the Crocus – Nature’s Poisons
Eating colchicine can lead to all manner of unpleasant symptoms, and more seriously can result in multiple organ failure and death. In short, you really don’t want to get autumn crocuses confused.
Despite its toxicity, colchicine has also been used as a treatment for gout due to its anti-inflammatory properties – usually when patients can’t take more standard anti-inflammatory painkillers. It’s another classic case of the dose making the poison, albeit one where there’s not a huge degree of difference: the dose required for therapeutic effects is not hugely distant from the dose at which toxicity is seen.
The good news is that true crocuses don’t contain colchicine. While eating bits of them still isn’t recommended – they still contain other compounds which, though they may not kill you, are still more than capable of kicking off unpleasant symptoms – saffron itself is safe to eat. Considering how many crocus plants it takes to make it, you’re probably still best off buying it at the supermarket.
The graphic in this article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. See the site’s content usage guidelines.
Información obtenida de https://www.compoundchem.com/2022/03/15/crocus/
To download a pdf of this article, visit http://cenm.ag/stimulants.
References used to create this graphic:
Oliver-Bever, B. “Why Do Plants Produce Drugs? Which Is Their Function in the Plants?” Q. J. Crude Drug Res. (1970). DOI: 10.3109/13880207009066221.
Spinella, Marcello. The Psychopharmacology of Herbal Medicine: Plant Drugs That Alter Mind, Brain, and Behavior. Cambridge, MA: MIT Press, 2001.
Wiart, Christophe. “Plants Affecting the Central Nervous System.” In Ethnopharmacology of Medicinal Plants: Asia and the Pacific, 57–153. Totowa, NJ: Humana Press, 2006.
A collaboration between C&EN and Andy Brunning, author of the popular graphics blog Compound Interest
If you haven’t seen the previous editions of this series, they’re available here: Part 1, part 2, and part 3. There’s also an edition looking at contemporary women in chemistry, and a graphic looking at the women of the periodic table. Additionally, there’s the mammoth ongoing project to highlight contemporary women in chemistry which currently features 170 entries and counting!
The text of this graphic is reproduced below for screenreaders.
Rona Robinson (1884-1962)
The first woman in the UK to gain a first-class degree in chemistry. She later carried out research on dyes and was also a campaigner for women’s suffrage.
Rebeca Gerschman (1903-1986)
The first scientist to suggest that oxygen free radicals damage cells and cause cell ageing. She was nominated for a Nobel Prize but died before being considered.
Ruby Hirose (1904-1960)
Carried out research on serums and antitoxins. Her work contributed to the development of an effective polio vaccine, leading to its near-eradication.
Mary Elliott Hill (1907-1969)
Thought to be the first African American woman to be awarded a master’s degree in chemistry. With her husband, Carl McClellan Hill, developed ketene synthesis.
Mildred Cohn (1913-2009)
Used nuclear magnetic resonance to study the reactions of enzymes and proteins in the human body, particularly focusing on the reactions of ATP.
Asima Chatterjee (1917-2006)
The first woman to receive a doctorate at an Indian university. Carried out research on plant-derived medicines, leading to anti-epileptic and anti-malarial drugs.
Katsuko Saruhashi (1920-2007)
Carried out research showing that seawater releases more carbon dioxide than it absorbs, and also identified radioactive isotopes in seawater due to nuclear testing.
Helen Murray Free (1923-2021)
Worked on the development of test strips for diseases, including urine analysis ‘dip and read’ test strips for UTIs, diabetes and kidney disorders.
Evangelina Villegas (1924-2017)
Worked with Surinder Vasal to improve the amino acid content of maize, making it more nutritious. They were awarded the World Food Prize for their work.
Alma Levant Hayden (1927-1967)
Amongst the first African American scientists to work at the US Food & Drug Administration, where she uncovered Krebiozen as a sham cancer treatment.
Bettye Washington Greene (1935-1995)
Researched latex and polymers at Dow Chemical, which led to several patents. She was the first black woman to work in a professional position at the company.
Margarita Salas (1935-2019)
Discovered an enzyme which can amplify DNA samples, making them large enough for analysis, with important applications in forensics and medical testing.
The graphic in this article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. See the site’s content usage guidelines.
Información obtenida de https://www.compoundchem.com/2022/03/08/iwd2022/
The graphic in this article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. See the site’s content usage guidelines.
Información sacada de https://www.compoundchem.com/2022/01/11/plant-milk/
In 2020, science news was dominated by COVID and vaccine development. In many ways, 2021 has been little different, but away from the virus we’re now overly familiar with there were plenty of other chemistry-related news stories. This graphic highlights a selection of them – see below for more details as well as links to related articles and studies.
The graphic in this article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. See the site’s content usage guidelines.
Informacion de https://www.compoundchem.com/2021/12/30/tyic2021/